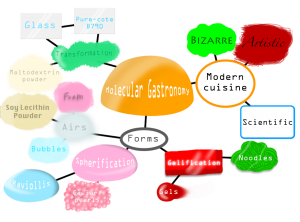
Molecular Gastronomy
The term, “Molecular and Physical Gastronomy,” was first coined in 1988 by Hungarian physicist Nicholas Kurti, and French physical chemist Hervé This. Molecular gastronomy is the science, and art of physically, and chemically transforming ingredients that occur in cooking. Spherification, gelification, and emulsification are just some of the many techniques to form caviar, raviolis, noodles, gels, ice creams, transparent wraps, and even foams, or airs. Transformations in ingredients can intensify the dining experience, appealing to taste, sight, and smell.
Spherification: The process of creating caviar, or ravioli-like spheres from liquids, or purees (ex. yogurt, sauces, fruit juice, soda) that are mixed with sodium alginate then bathed in a cold water solution containing calcium chloride (for fruit juices, or liquids without calcium). Another method of spherification is, “reverse spherification” that involves mixing calcium lactate with liquids that already contain calcium, or high levels of acid/alcohol into an alginate water bath. A third recent method is, “frozen reverse spherification” that involves pre-freezing the liquid spheres, but all three methods result in either popping caviar/pearls, and raviolis that explode with flavor from the lightest pressure.
Gelification: The process of turning liquids into gelatinous forms (noodles, sheets, or gels) with Agar-agar (noodles with a glossy appearance), Carrageenan (for gels that appear brittle and firm), Gellan gum (for firmer gels), Methylcellulose (used as a gel thickening agent), and Pectin (used as a gelling sugar for jams, spreads, and sweet sauces). Gelification occurs when macromolecules are bound with large amounts of water, and can be frozen, or chilled to create gelatinous forms.
Emulsification: The process of creating foams through the use of incorporating emulsification agents (soy lecithin) while whisking swiftly to create air bubbles, or creating gels, ice creams, sauces, and dressings through a process of heating, and incorporating methylcellulose into a mix of other liquids, and dairy products.
The Science:
Emulsification process: Emulsification is a process that is used in several everyday products, like chocolate, milk, butter, etc. Under microscopic vision, thousands of small droplets are dispersed in a second liquid substance. These substances are oil, and water that have been mixed with an emulsifying agent. The method of emulsification in molecular gastronomy is taking a liquid trapping air bubbles to create a foam; this is done by vigorously whisking air bubbles into a liquid, and is stabilized with an emulsification agent. Common emulsifying agents include milk and egg protein, cream fat, bread starch, and gelatin; new discoveries have proven that soy lecithin, and methylcellulose are also sufficient emulsifying agents.
The lecithin molecule positions itself around air bubbles, which inflate their hydrophilic portion towards the water. The air bubbles’ surface is surrounded by lecithin molecules, which prevents water from escaping from the liquid. The process of emulsification with methylcellulose is used for more texture based dishes, and formations (ex. ice creams, sauces, and dressings). Methyl cellulose is a chemical compound taken from vegetable cellulose through heating with a caustic solution, and treatment with methyl chloride. The end product is a white odorless powdery substance that swells up in the presence of liquid.
Methylcellulose is an effective agent in preventing the formation of ice crystals in foods which need frequent refrigeration, keeping food fresher. In molecular gastronomy it is often used as a gelling agent. To use methylcellulose, it must first be hydrated in cold liquid with a dosage of about 1g to 20g per liter, depending on the desired outcome. The solution must then be stirred, or shaken, and left to rest.
Creating the foam: https://www.youtube.com/watch?v=hsf-BUKViwg
Gelification: This process can be accomplished with common gelling agents such as corn starch, tapiocas, flour, eggs, and gelatin. However, in molecular gastronomic terms, the process involves a rearrangement of the molecules that align and attach themselves until they form a network that traps the liquid. This network looks like meshes of a net that keep all of the particles in suspension, preventing their aggregation and the collapse of the structure, and can be done with non-traditional gelling agents called, hyrdocolloids. The process of gelification is performed in three stages:
Dispersion: An essential step for the formation of a gel and for the thickening of a preparation. An improperly dispersed gelling agent will stick together and form lumps that will alter the subsequent formation of the gel. Dispersion must allow the gelling agent molecules to be completely surrounded by water by separating the powder particles. For several hydrocolloids (agar-agar, carrageenan, sodium alginate, gellan gum), this requires vigorous stirring of the mixture with cold water.
Hydration: Allows water to penetrate inside the hydrocolloid molecules, which then facilitate reactions, as it is surrounded by water and suspended in the solvent. This step can be done by gradually heating or chilling the liquid. Agar-agar, carrageenans, some gelatins and gellan gum require heating to hydrate. Alginate hydration requires cooling; the process is described in detail in the section on spherification.
Formation: Occurs after hot hydration, when the temperature drops to a gelling temperature that is specific to each additive. Although some gels are formed before reaching room temperature, others require refrigeration.
Gelification example video: http://www.youtube.com/watch?v=vt34q4TVFyY
Spherification: A process that transforms liquids, or purees into spherical shapes (raviolis, or caviar/pearls) that burst in the mouth. The additive used is sodium alginate, and just like in the gelling process using carrageenans or gellan gum, the presence of ions is essential for the formation of the gel. In the case of a sodium alginate gel, the presence of calcium ions is required so that the long alginate molecules can align and bind to finally form a gel. Alginate reacts with any calcium that naturally occurs or that has been added to the ingredient to be spherified. For example, we could make a pudding by simply adding sodium alginate to a preparation of milk and sugar, as milk is naturally rich in calcium.
Applying this principle, we can precisely control the moment when the calcium, and alginate come into contact and thereby diversify the liquids to be gelled, and the forms obtained.






February 22nd, 2023 at 6:50 pm
I think this is a really good article. You make this information interesting and engaging. You give readers a lot to think about and I appreciate that kind of writing. Healthy Marriage
July 3rd, 2023 at 11:47 am
This article is a really awesome article. It’s interesting and informative article. myfedloan
July 5th, 2023 at 6:59 am
Can you use the pictures in the article? We will indicate the source https://www.marykayintouch.website/
July 18th, 2023 at 6:01 am
By applying scientific principles to cooking, molecular gastronomy opens up new possibilities for chefs to explore flavors, textures, and presentations. It pushes the boundaries of traditional culinary techniques, allowing for creative and artistic expression in the kitchen. myccpay
July 29th, 2023 at 5:45 am
That was so great. https://mcdvoice.me/
August 7th, 2023 at 10:40 am
Emulsification is a process that is used in several everyday products, like chocolate, milk, butter. TellPopeyes
September 5th, 2023 at 5:53 pm
That was so great. https://marykayintouch.online/
September 14th, 2023 at 9:13 am
Thanks for sharing. Follow My Health
September 28th, 2023 at 5:56 am
Thanks for sharing. It’s so interesting. Blackboard UTSA
October 11th, 2023 at 7:42 am
thanks for sharing this http://www.mycvshr.com
October 18th, 2023 at 5:16 pm
Pretty nice post. I just stumbled upon your weblog and wanted to say that I have really enjoyed browsing your blog posts. After all I’ll be subscribing to your feed and I hope you write again soon! لاند كروزر 2023
October 23rd, 2023 at 3:46 pm
I truly enjoyed reading it. This subject offered by you is very helpful and accurate. https://mykohlscards.online/
October 30th, 2023 at 11:57 am
There is so much in this article that I would never have thought of on my own. Your content gives readers things to think about in an interesting way. Thank you for your clear information. Strapless Bra
October 31st, 2023 at 3:45 pm
I’ve read some good stuff here. Definitely worth bookmarking for revisiting. I surprise how much effort you put to create such a great informative website. sexy lingerie
October 31st, 2023 at 3:46 pm
I admire what you have done here. I like the part where you say you are doing this to give back but I would assume by all the comments that this is working for you as well. Sportswear
November 2nd, 2023 at 12:53 pm
You have a real ability for writing unique content. I like how you think and the way you represent your views in this article. I agree with your way of thinking. Thank you for sharing. nighty for women
November 15th, 2023 at 3:38 am
Thanks for sharing this information is really helpful.
Appreciated. Upsers
January 3rd, 2024 at 2:56 am
This is fascinating to me. Molecular Gastronomy is something my cousin is really into, but I never realized how much there is to learn about this. I thought my online survey hobby was cool, but this is even better.
January 4th, 2024 at 9:54 pm
Thanks for sharing this information, It was a great blog keep sharing stuff like this. Joinpd
January 4th, 2024 at 10:00 pm
Thanks for the great stuff, Keep sharing posts like this they really help users educate themselves. liteblue
February 26th, 2024 at 8:09 pm
I like this type of blogs they are very helpful and informational. recep tify
February 26th, 2024 at 8:11 pm
Your content gives readers things to think about in an interesting way. Thank you for your clear information. postalease liteblue
March 1st, 2024 at 12:08 am
Thank you for sharing this knowledge. Education never ends. applydiscoverit.com 4 word code